PASSIVATION
The process Passivation is removal of exogenous iron or iron compounds from the surface of a stainless steel by means of a chemical dissolution, most typically by a treatment with an acid solution that will remove the surface contamination but will not significantly affect the stainless steel itself for the purpose of enhancing the spontaneous formation of the protective passive film.
Why Passivate Stainless Steel?
Passivation is a post-fabrication best practice for newly-machined stainless steel parts and components. Benefits include:
- Chemical film barrier against rust
- Extended life of the product
- Removal of contamination from product surface
- Reduced need for maintenance.
How Does Passivation Work?
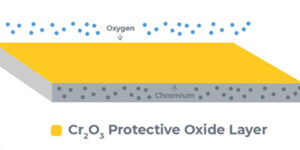
Stainless steel is an iron-based alloy, typically composed of iron, nickel and chromium. Stainless steel derives its corrosion-resistant properties from the chromium content. Chromium, when exposed to oxygen (air), forms a thin film of chromium oxide that covers the stainless steel surface and protects the underlying iron from rusting. The purpose of passivation is to augment and optimize formation of the chromium oxide layer.
- Immersion of stainless steel in an acid bath dissolves free iron from the surface while leaving the chromium intact. The acid chemically removes the free iron, leaving behind a uniform surface with a higher proportion of chromium than the underlying material.
- Upon exposure to oxygen in the air after the acid bath, the stainless steel forms the chromic oxide layer over the next 24 to 48 hours. The higher proportion of chromium at the surface allows for the formation of a thicker, more protective chromium oxide layer. Removal of free iron from the surface removes opportunities for corrosion to start.
- The resulting passive layer provides a chemically non-reactive surface that protects against rust.
- We use Loop-Circulation / direct process to do Passivation.
